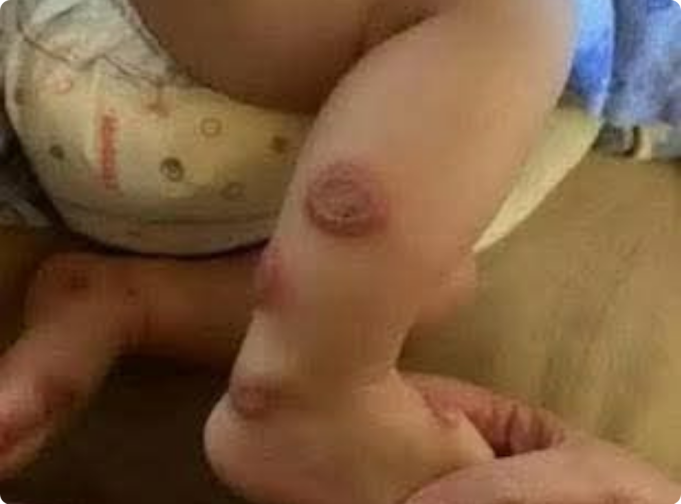
The following case illustrates a rare but significant cutaneous reaction to a newly initiated inhaled medication—a condition that underscores the importance of prompt recognition and referral in primary care settings.
Case Presentation
A 55-year-old woman with a history of hypertension and chronic obstructive pulmonary disease (COPD) presented to her primary care clinic with acute skin lesions. Her regular medications included enalapril (for six years) and inhaled formoterol (for two years). She reported no known drug allergies, was a current smoker (10 cigarettes/day), and denied recent changes in diet, cosmetic use, or illness.
Due to worsening COPD symptoms, her pulmonologist recently modified her inhalation regimen: formoterol was discontinued, and she was started on a fixed-dose combination of indacaterol and glycopyrronium via inhaled capsules.
On the second day of this new therapy, she developed painful, erythematous patches on her cheeks and neck, accompanied by low-grade fever (Fig. 1). She reported no recent upper respiratory symptoms, and although she had been exposed to sunlight, she used appropriate sun protection.
Clinical Management
Given the acute onset, atypical distribution, and systemic symptoms, an urgent referral to Dermatology was initiated. The dermatology team recommended immediate discontinuation of the indacaterol/glycopyrronium inhaler and ordered further diagnostic workup, including:
- Skin biopsy
- Complete blood count (CBC)
- Autoimmune serology (ANA, anti-dsDNA)
- Lupus anticoagulant testing
- Infectious disease serology
Empiric treatment with oral corticosteroids was started.
Outcome and Laboratory Findings
Within 24–48 hours of stopping the inhaler and initiating corticosteroids, the patient’s skin lesions markedly improved, with reduced erythema and resolution of pain.
Laboratory results revealed:
- Leukocytosis with neutrophilia
- Positive autoantibodies and lupus anticoagulant
- Negative infectious serology
These findings, combined with the temporal association with the new medication and rapid clinical improvement upon withdrawal, strongly suggest a drug-induced hypersensitivity reaction, possibly mimicking a lupus-like or neutrophilic dermatosis.
Key Takeaway for Primary Care
This case highlights that inhaled medications—often perceived as low-risk due to localized delivery—can trigger systemic immune-mediated reactions. Primary care providers should maintain a high index of suspicion for new-onset rashes following medication changes, even with non-oral drugs. Early recognition, prompt discontinuation of the suspected agent, and timely specialist referral are critical to preventing progression and ensuring optimal outcomes.
Vigilance in pharmacovigilance remains essential—even when the route of administration seems innocuous.